Go back
Sakura Finetek USA complies with the FDA UDI (Unique Device Identification) requirements and completes the implementation of the new label design and adds GS1 (Global Standards 1) barcodes to product labels.
Innovation
February 05, 2021
America
TORRANCE, CA – Sakura Finetek USA, Inc. today announced the completion of implementing the FDA UDI compliant labels and the new corporate identity for its products.
“As part of our continuous improvement program, we have made several changes to the product labels to include more valuable information for our customers, global distribution and manufacturing. The major changes relate to the design getting aligned with our new corporate identity (CI) guidelines and the inclusion of the GS1 barcode, that addresses new requirements of the FDA UDI for Class I IVD devices enforced for compliance by September 2022”, said Solmaz Shaida, Director of Quality Assurance and Regulatory Affairs.
All product labels have 2 or 3 barcodes
The most important barcode is the GS1 barcode (in most cases 2D barcode), followed by human readable code; this barcode includes the GTIN (Global Trade Identification Number), which contains of primary code (static portion) also known as DI (Device Identifier) such as: the labeler name, the product code, the product name and description, and may also include a web-link (URL); in addition to secondary code (dynamic portion) also known as PI (Production Identifier) as applicable such as: an expiration date and LOT number (for reagents and consumables) or a serial number (for instruments).
The other barcode (in most cases) is the linear HIBC (Health Industry Bar Code) barcode, which includes a company identifier, the product code and the packaging level.
The GS1 and HIBC barcodes are used from the point of manufacturing, over distribution, to inventory management or retrieval of product-related information.
Sakura Finetek USA utilizes the GS1 as Accredited Issuing Agency for obtaining the UDI and submission to the FDA GUDID (Global Unique Identification Database). Please refer to Access GUDID (
https://accessgudid.nlm.nih.gov) for more details.
The final barcode is proprietary barcode. “This barcode is used by our instrumentation to verify product identity, drive the instruments to proper use and enrich the information provided on-board. Examples of the Sakura Finetek barcodes are those used on the Tissue-Tek Genie
® Advanced System and Tissue-Tek Prisma
® Automated Stainer product lines” said Erico von Bueren, Director of Marketing.
The design of the label changed
When comparing the two labels below, the new corporate identity guidelines have been applied to the label (right) including the change of the color for the product name to the Sakura Finetek blue, change to a more legible font throughout the entire label, and the addition of the GS1 2D barcode.
The line consisting of slanted rectangles in red under the brand name (left) has been removed and replaced with the Sakura Finetek logo with a red bar (right):
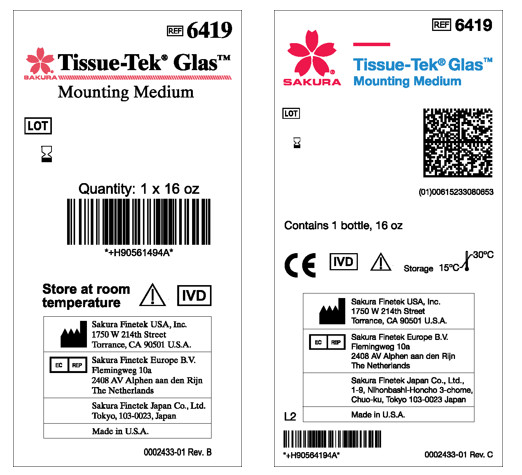
In some cases, we redesigned labels to be printed only in black to accommodate some automated production requirements. The next 2 labels show the previous design using the Sakura Finetek logo in red (left) and the new one with the Sakura Finetek logo in black (right):

For some products we now include three barcodes to not only enable the use of the HIBC and GS1 barcode throughout the supply chain (left), but to also include a 2D-data-matrix barcode used by the instruments to register consumables used on them, like the label affixed to a bottle of the Tissue-Tek Genie Dewax Solution (right):
 About Sakura Finetek USA, Inc.
About Sakura Finetek USA, Inc.
With the U.S. office based in Torrance, California, Sakura Finetek is the global leader in
continuous innovation for pathology by providing integrated solutions for anatomic pathology and patients through best-in-class innovation, quality and customer care.
With a strategic focus on end-to-end automation, Sakura Finetek continues to lead the industry in the development and commercialization of automated histology instrumentation and consumables for anatomic pathology.
Sakura Finetek solutions dramatically increase efficiency, standardize results, and enable pathology laboratories to more simply manage their daily workload while significantly impacting patient care. Visit
www.sakuraus.com to learn more.
Erico von Bueren
Director of Marketing
Sakura Finetek USA, Inc.
1750 W. 214
th St.
Torrance, CA 90501
evonbueren@sakuraus.com